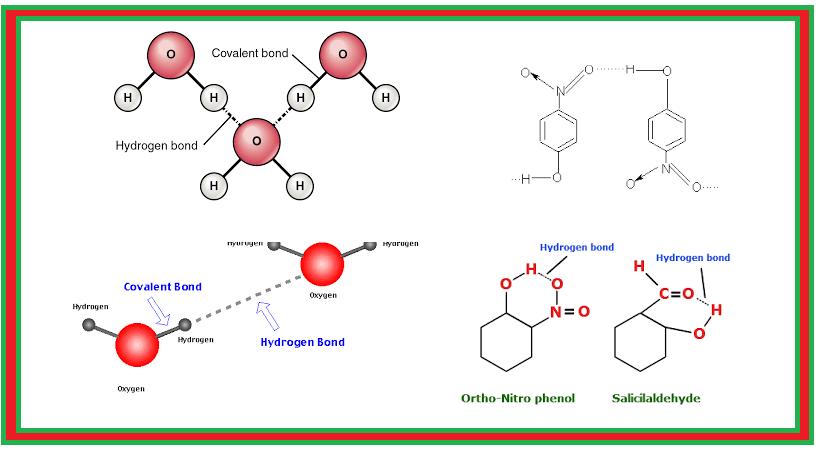
Hydrogen Bonding :
`=>` Nitrogen, oxygen and fluorine are the higly electronegative elements.
● When they are attached to a hydrogen atom to form covalent bond, the electrons of the covalent bond are shifted towards the more electronegative atom.
● This partially positively charged hydrogen atom forms a bond with the other more electronegative atom.
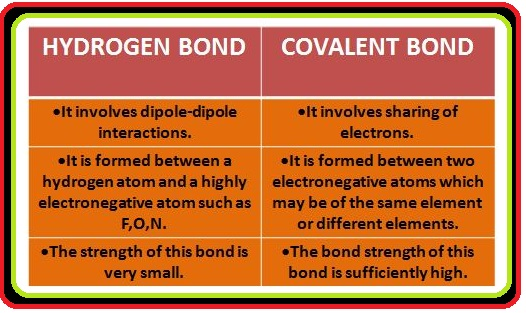
● This bond is known as hydrogen bond and is weaker than the covalent bond.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Whenever a molecule contains a hydrogen atom linked to a highly electronegative atom(like N,O or F), this atom attracts the shared pair of electrons more and so this end of the molecule becomes slightly negative while the other end becomes slightly positive. The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This bond is called hydrogen bond
`text(Example :)` In `HF` molecule, the hydrogen bond exists between hydrogen atom of one molecule and fluorine atom of another molecule as depicted below :
`--- H^(delta +) - F^(delta-)`
● Here, hydrogen bond acts as a bridge between two atoms which holds one atom by covalent bond and the other by hydrogen bond.
`=>` Hydrogen bond is represented by a dotted line (`– – –`) while a solid line represents the covalent bond.
`=>` Thus, hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (`F`, `O` or `N`) of another molecule.
● When they are attached to a hydrogen atom to form covalent bond, the electrons of the covalent bond are shifted towards the more electronegative atom.
● This partially positively charged hydrogen atom forms a bond with the other more electronegative atom.
● This bond is known as hydrogen bond and is weaker than the covalent bond.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
Whenever a molecule contains a hydrogen atom linked to a highly electronegative atom(like N,O or F), this atom attracts the shared pair of electrons more and so this end of the molecule becomes slightly negative while the other end becomes slightly positive. The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This bond is called hydrogen bond
`text(Example :)` In `HF` molecule, the hydrogen bond exists between hydrogen atom of one molecule and fluorine atom of another molecule as depicted below :
`--- H^(delta +) - F^(delta-)`
● Here, hydrogen bond acts as a bridge between two atoms which holds one atom by covalent bond and the other by hydrogen bond.
`=>` Hydrogen bond is represented by a dotted line (`– – –`) while a solid line represents the covalent bond.
`=>` Thus, hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (`F`, `O` or `N`) of another molecule.